Identify the product of the following reaction. The products for this last reaction will be ethylhexanoate and water.
8 Esters And Formation Of Esters Alcohol Carboxylic Acid And Esters
The chemical reaction that takes place during the formation of the ester is called esterification.
. Identify the product of the following reaction. Acylation of Ketones Reaction type. AThe reactions are irreversible.
For example in the following reaction which is ester is the major product. In the general form above an alcohol red and a carboxylic acid. Make a list of the odours you were able to detect and the ester responsible for.
--Fatty acid molecules with single bonds between all carbons are _____. Bettelheim Chapter 194 Problem 192P. Mention all limitations and challenges in this experiment.
Identify the correct statement about base hydrolysis of esters. Esterification is a process or a general name for a chemical reaction in which two reactants alcohol and an acid form an ester as the reaction product. Ketone enolates can be acylated with non-enolisable esters ie.
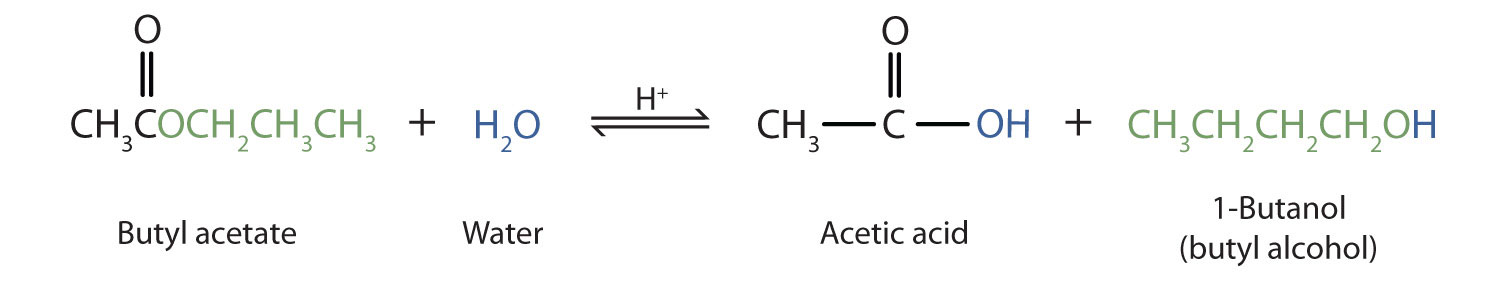
Literally splitting with water. When alcohol reacts with symmetrical acid anhydride to form ester. CThe process is saponification.
Identify the product of the following reaction. Heat HCI ОН ОН H O нс НО CH. The forward reaction is.
The hydrolysis of esters can be catalyzed by either an acid or a base with somewhat different results. Use the pop-up menus to identify the total number of aldol products that each of the following reactions can produce. The formula for carboxylic acid esters is RCOOR where R and R are any organic combining groups that are prepared again by the reaction of alcohols and carboxylic acids in the presence.
CThe process is saponification. An ester that lacks a-hydrogens. --A chemical reaction in which an ester is converted into a new type of ester is a _____.
Double bond between Br and CH3. Most commonly the base would be the alkoxide RO- matching the alcohol portion of the ester Remember enolates are good nucleophiles and the ester carbonyl C are electrophilic and. Then join the two.
Also note that this technique is for reactions between alcohols and carboxylic acids. The reaction of a substance with water. You remove OH group from the alcohol and from the carboxylic acid.
Select all that are true. In the formation of an ester one oxygen atom comes from the alcohol molecule while the carbonyl group comes from the carboxylic acid. To make short-chain esters such as ethyl ethanoate CH3COOCH2CH3 heat ethanol and ethanoic acid with a strong concentrated acid catalyst and distill off the product ie the ester.
We have the following reaction But if the acid anhydride used is unsymmetrical how can we identify the major product. A series of derivatisation reactions between p-t-butyl calix4arene and ethyl bromoacetate were carried out in order to prepare 13 diester substituted calix4arene. Ester is obtained by an esterification reaction of an.
Identify the esters formed in relation to the odours smelt. Identify the products of the reaction that will be carried out in biodiesel synthesis lab. CH 3 COOH C 2 H 5 OH CH 3 COOC 2 H 5 H 2 O.
The direct method for the preparation of esters is the reaction between the carboxylic acid and the alcohol For example the ethyl acetate ester is obtained by the reaction of acetic acid and ethyl alcohol. Identify the sole product of the following reaction. I tried searching the internet for the mechanism of this reaction but was able to.
AThe reactions are irreversible. Identify the reagent s A - I you would use to accomplish each of the following transformations. The ester has the lowest boiling point out of all the substances involved because it cannot form hydrogen bonds with itself unlike alcohols and carboxylic acids.
Esterification is the process of combining an organic acid RCOOH with an alcohol ROH to form an ester RCOOR and water. The ester is heated with a. Identify the name of the carboxylic acid shown below.
Or a chemical reaction resulting in the formation of at least one ester product. Draw the structural formulae of the formation of the two esters. Draw the structure of an alkyl chloride that will undergo an E2 elimination to yield only the indicated alkene.
Mass spectral data obtained from direct injection of samples indicated that the reactions were rich in. The ester product was extracted into dichloromethane and then shaken with saturated sodium chloride solution. Preparation of ethyl acetate ester.
The ester product formed from the reaction of ethanol with ethanoic acid acetic acid would be. This is known because it is possible to label using radioactive nuclides the atoms of the reactants and see where they end up in the products. For the last reaction you will have to use the same procedure as with the first reaction I gave.
Acidic hydrolysis is simply the reverse of esterification. We have step-by-step solutions for your textbooks written by Bartleby experts. Identify the name of carboxylic acid shown below.
Textbook solution for Introduction to General Organic and Biochemistry 11th Edition Frederick A. Dnone of the above. Identify a correct product of the reduction of propanoic acid from the structures below.
BThe reactions are reversible. This reaction is reversible where the formed ester is hydrolyzed to the acid and. Identify the ester product of the reaction.
This reaction will not proceed as written ortho-dibromobenzene D. The tetrahedral intermediate collapses with a loss of water to form ester.

Question Video Identifying The Products Of An Ester Hydrolysis Reaction Nagwa

Lesson Explainer Properties Of Esters Nagwa

2 10 Reactions Of Esters Chemistry Libretexts

8 Esters And Formation Of Esters Alcohol Carboxylic Acid And Esters
0 Comments